
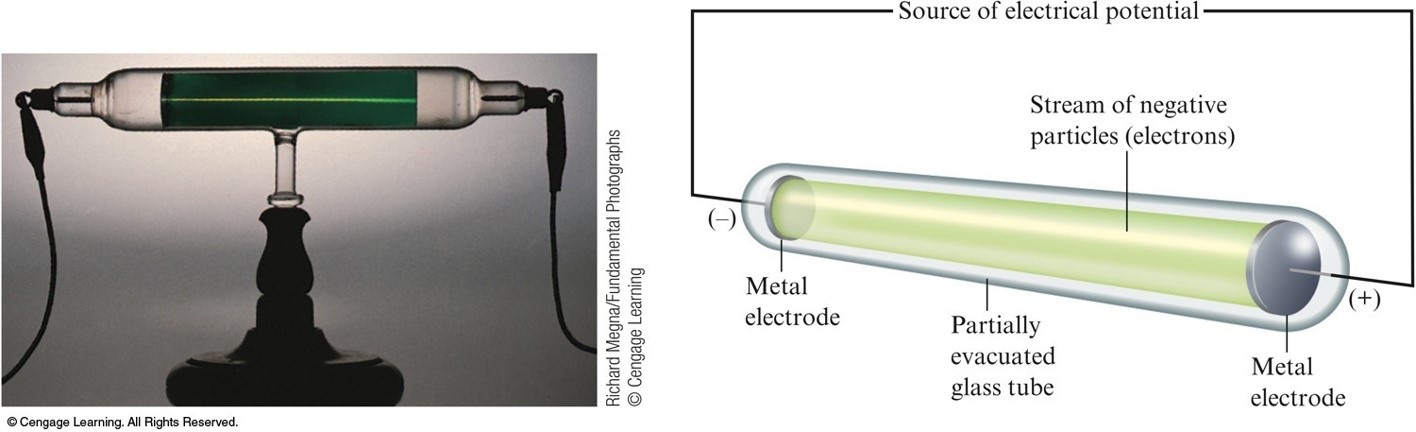
In 1838, Michael Faraday applied a high voltage between two metal electrodes at either end of a glass tube that had been partially evacuated of air, and noticed a strange light arc with its beginning at the cathode (negative electrode) and its end at the anode (positive electrode). Glow discharge in a low-pressure tube caused by electric current. These are used in cathode-ray tubes, found in televisions and computer monitors, and in electron microscopes. High speed beams of cathode rays can also be steered and manipulated by electric fields created by additional metal plates in the tube to which voltage is applied, or magnetic fields created by coils of wire ( electromagnets). The triode vacuum tube developed between 19 was the first electronic device that could amplify, and is still used in some applications such as radio transmitters. This is the principle used in vacuum tubes to amplify electrical signals. Thus, a small voltage on the grid can be made to control a much larger voltage on the anode. The amount of current that gets through to the anode depends on the voltage on the grid. The electric field of the wires deflects some of the electrons, preventing them from reaching the anode. The current in a beam of cathode rays through a vacuum tube can be controlled by passing it through a metal screen of wires (a grid) between cathode and anode, to which a small negative voltage is applied. After the electrons strike the back of the tube they make their way to the anode, then travel through the anode wire through the power supply and back through the cathode wire to the cathode, so cathode rays carry electric current through the tube. Researchers noticed that objects placed in the tube in front of the cathode could cast a shadow on the glowing wall, and realized that something must be traveling in straight lines from the cathode. Cathode rays are invisible, but their presence was first detected in these Crookes tubes when they struck the glass wall of the tube, exciting the atoms of the glass and causing them to emit light, a glow called fluorescence. The voltage applied between the electrodes accelerates these low mass particles to high velocities. They travel in parallel lines through the empty tube. Since the electrons have a negative charge, they are repelled by the negative cathode and attracted to the positive anode. The increased random heat motion of the filament knocks electrons out of the surface of the filament, into the evacuated space of the tube. Modern vacuum tubes use thermionic emission, in which the cathode is made of a thin wire filament which is heated by a separate electric current passing through it.
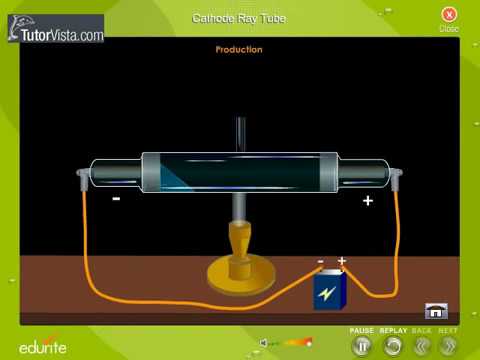
The positive ions were accelerated by the electric field toward the cathode, and when they collided with it they knocked electrons out of its surface these were the cathode rays.
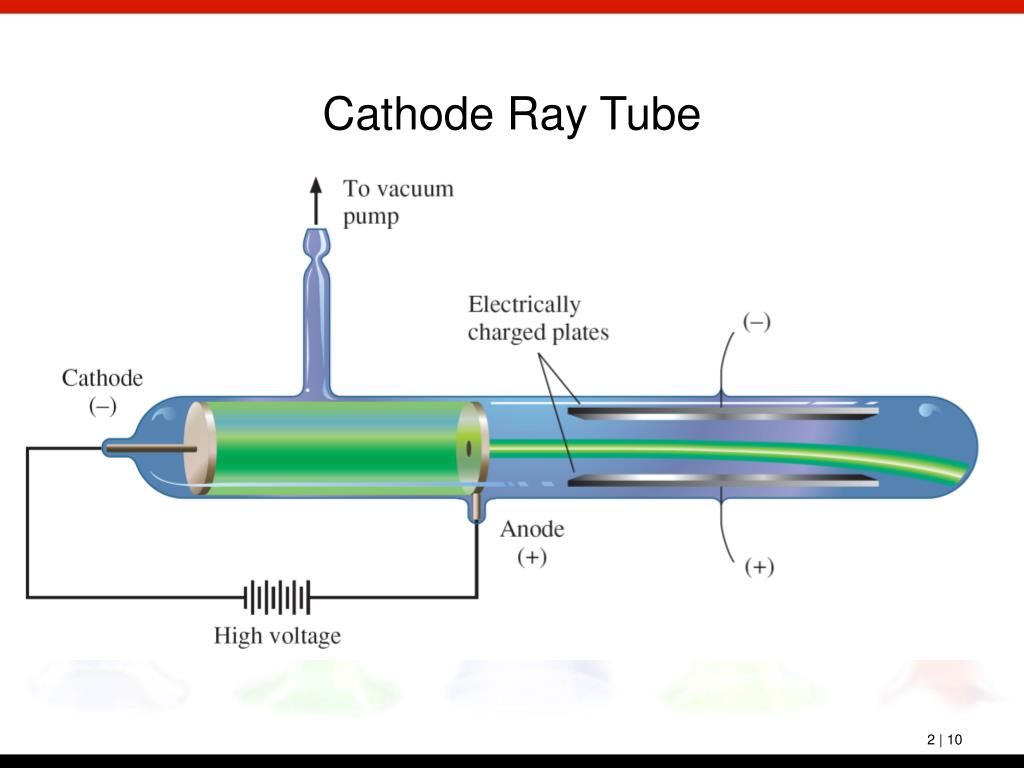
In the early experimental cold cathode vacuum tubes in which cathode rays were discovered, called Crookes tubes, this was done by using a high electrical potential of thousands of volts between the anode and the cathode to ionize the residual gas atoms in the tube. To release electrons into the tube, they first must be detached from the atoms of the cathode. The Maltese cross has no external electrical connection.Ĭathode rays are so named because they are emitted by the negative electrode, or cathode, in a vacuum tube. Cathode-ray tubes (CRTs) use a focused beam of electrons deflected by electric or magnetic fields to render an image on a screen.Ī diagram showing a Crookes tube connected to a high voltage supply. Thomson showed that cathode rays were composed of a previously unknown negatively charged particle, which was later named the electron. They were first observed in 1859 by German physicist Julius Plücker and Johann Wilhelm Hittorf, and were named in 1876 by Eugen Goldstein Kathodenstrahlen, or cathode rays. If an evacuated glass tube is equipped with two electrodes and a voltage is applied, glass behind the positive electrode is observed to glow, due to electrons emitted from the cathode (the electrode connected to the negative terminal of the voltage supply). Cathode rays are normally invisible in this demonstration with a Teltron tube, enough gas has been left in the tube that the gas atoms luminesce when struck by the fast-moving electrons.Ĭathode rays ( electron beam or e-beam) are streams of electrons observed in discharge tubes. A beam of cathode rays in a vacuum tube bent into a circle by a magnetic field generated by a Helmholtz coil.


 0 kommentar(er)
0 kommentar(er)
